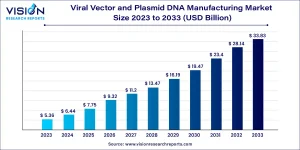
The global viral vector and plasmid DNA manufacturing market size was valued at USD 6.44 billion in 2024 and is projected to reach USD 33.83 billion by 2033, growing at a CAGR of 20.23%.
Understanding Viral Vectors and Plasmid DNA – What They Are and Why They Matter
Viral vectors and plasmid DNA are fundamental tools in modern biotechnology and medicine. They are used to deliver genetic material into cells to treat diseases, create vaccines, and develop advanced therapies. Viral Vectors are viruses that have been modified to carry therapeutic genes without causing disease. They act as delivery vehicles, transporting the gene of interest into target cells. Common viral vectors include adenoviruses, lentiviruses, and adeno-associated viruses (AAVs). These vectors are crucial for gene therapy, where faulty genes in patients are replaced or corrected.
Plasmid DNA is small, circular DNA that exists independently of chromosomal DNA. It is commonly used in laboratories for research and as a key component in vaccines, especially mRNA vaccines. Plasmid DNA can be easily manipulated, making it an efficient and safe tool for producing therapeutic proteins and vaccines.
Viral Vector and Plasmid DNA Manufacturing Market Overview
The viral vector and plasmid DNA manufacturing market is rapidly emerging as a cornerstone of modern biotechnology and biopharmaceutical development. These technologies form the backbone of gene therapies, vaccines, and advanced therapeutics, providing precise tools for delivering genetic material into target cells. The market has witnessed significant interest from pharmaceutical companies, research institutions, and contract manufacturing organizations due to the rising demand for personalized medicine, immunotherapies, and large-scale vaccine production.
Viral vectors, such as adenoviruses, lentiviruses, and adeno-associated viruses (AAVs), are widely used for gene delivery, while plasmid DNA serves as a key platform for mRNA vaccines and therapeutic applications. The convergence of innovations in cell and gene therapy, coupled with regulatory support and technological advancements, continues to fuel the expansion of this critical market segment.
Access Your Sample Report Today: https://www.visionresearchreports.com/report/sample/41584
Viral Vector and Plasmid DNA Manufacturing Market Growth
The viral vector and plasmid DNA manufacturing market is on an accelerated growth trajectory, primarily driven by the rising adoption of gene and cell therapies. Biopharmaceutical companies are increasingly investing in scalable manufacturing processes to meet the growing demand for high-quality viral vectors and plasmid DNA. The integration of advanced purification techniques, automated production systems, and process optimization has enhanced manufacturing efficiency and reduced production costs, facilitating broader accessibility for research and clinical applications.
In addition, the COVID-19 pandemic has underscored the importance of rapid vaccine development, highlighting plasmid DNA and viral vector platforms as essential tools for next-generation therapeutics. Collaborations between biotech firms and academic institutions are further accelerating innovation, leading to improved yields, higher vector potency, and the development of novel delivery systems that strengthen the market’s long-term growth prospects.
Viral Vector and Plasmid DNA Manufacturing Market Trends
- Rise of Gene and Cell Therapies: Increasing approvals and pipeline expansion for gene therapies are creating significant demand for viral vector production. Companies are exploring novel viral serotypes and optimized plasmid constructs to improve therapeutic outcomes.
- Adoption of Single-Use Technologies: Single-use bioreactors and modular manufacturing solutions are gaining popularity due to their flexibility, reduced contamination risk, and lower capital expenditure.
- Expansion of Contract Manufacturing: Contract Development and Manufacturing Organizations (CDMOs) are expanding their capabilities to meet the increasing outsourcing demand for plasmid DNA and viral vector production, driving market efficiency.
- Regulatory Harmonization: Regulatory authorities are providing clearer guidelines for clinical-grade vector production, facilitating smoother approvals and fostering confidence among developers and investors.
How They Work – A Simple Explanation of Gene Delivery and DNA Use
The working principle of viral vectors and plasmid DNA is based on delivering genetic instructions to cells.
- Viral Vectors: Scientists remove harmful parts of the virus and insert a therapeutic gene. When the modified virus enters the patient’s cells, it delivers the gene, which then produces a protein to correct or treat a disease. This process is widely used in gene therapy, where it can permanently or temporarily correct genetic defects.
- Plasmid DNA: Plasmids carry the gene of interest and are introduced into cells using methods like electroporation, liposomes, or nanoparticle carriers. Once inside, the cells read the genetic instructions from the plasmid and produce the desired protein. Plasmid DNA is essential in vaccines, where it instructs cells to make viral proteins that trigger an immune response without causing infection.
Viral Vector and Plasmid DNA Manufacturing Market Dynamics
Drivers
Key drivers include the growing prevalence of genetic disorders, the surge in cell and gene therapy applications, and the increasing demand for vaccine using plasmid DNA or viral vectors. Advances in biotechnology and manufacturing processes are making large-scale production more feasible and cost-effective.
Opportunities
Emerging markets, strategic collaborations, and technological innovations such as continuous manufacturing and automated purification systems present significant opportunities. The expanding pipeline of gene therapies and vaccine programs worldwide will continue to fuel market growth.
Challenges
Challenges include complex manufacturing processes, high production costs, stringent regulatory requirements, and the need for skilled workforce and infrastructure. Additionally, ensuring product stability, potency, and scalability remains a critical hurdle for developers.
Want custom data? Click here: https://www.visionresearchreports.com/report/customization/41584
COVID-19 Impact on Viral Vector and Plasmid DNA Market
The COVID-19 pandemic had a transformative impact on the viral vector and plasmid DNA market, highlighting the critical role of these technologies in rapid vaccine development and pandemic preparedness.
- Acceleration of Vaccine Development: Viral vectors and plasmid DNA became key platforms for COVID-19 vaccines. Adenovirus-based vectors and DNA platforms enabled rapid design and deployment, allowing vaccines to reach clinical trials and regulatory approval in record time.
- Increased Demand for Manufacturing Capacity: The pandemic exposed limitations in large-scale production, driving investments in advanced manufacturing facilities, automated production, and contract development partnerships to meet urgent global demand.
- Innovation and Collaboration: COVID-19 fostered unprecedented collaboration between biotech companies, governments, and academic institutions. This accelerated improvements in vector design, purification methods, and scalable plasmid DNA production.
- Market Awareness and Adoption: The success of viral vector and plasmid DNA vaccines enhanced confidence in these technologies for other applications, such as gene therapies, immunotherapies, and future vaccines against emerging infectious diseases.
Real-Life Applications – Where and How They Are Used in Medicine
Viral vectors and plasmid DNA are no longer just research tools—they are actively shaping modern medicine:
- Gene Therapy: Patients with genetic disorders, such as spinal muscular atrophy or hemophilia, receive gene therapy using viral vectors to restore or replace missing proteins. These treatments can dramatically improve quality of life or even cure previously untreatable diseases.
- Vaccines: Plasmid DNA and viral vectors have been central to rapid vaccine development. For instance, some COVID-19 vaccines relied on viral vectors to deliver instructions for producing viral proteins, enabling the immune system to recognize and fight the virus.
- Cancer Immunotherapy: Modified viral vectors can deliver genes that stimulate the immune system to attack tumors, offering new hope for cancers resistant to conventional treatments.
- Rare Diseases: AAV-based gene therapy has been used to treat rare genetic disorders, providing long-term therapeutic benefits with a single treatment in some cases.
Case Study: Success Story – Example of a Breakthrough in Production or Therapy
Case Study: AAV Gene Therapy for a Rare Disease
A biotechnology company focused on treating rare genetic disorders implemented an advanced production system for AAV-based gene therapy. By using single-use bioreactors and optimized purification processes, the company was able to significantly increase viral vector yield while maintaining high quality and safety standards.
The therapy targeted a rare inherited disorder causing vision loss. Patients who received the treatment experienced significant improvements in vision, highlighting both the effectiveness of the therapy and the importance of advanced manufacturing processes.
Read More: https://www.heathcareinsights.com/biomedical-refrigerators-and-freezers-market/
Top Companies in Viral Vector and Plasmid DNA Manufacturing Market
- Merck KGaA
- Lonza
- FUJIFILM Diosynth Biotechnologies
- Thermo Fisher Scientific
- Cobra Biologics
- Catalent Inc.
- Wuxi Biologics
- Takara Bio Inc.
- Waisman Biomanufacturing
- Genezen laboratories
- Batavia Biosciences
- Miltenyi Biotec GmbH
- SIRION Biotech GmbH
- Virovek Incorporation
- BioNTech IMFS GmbH
- Audentes Therapeutics
- BioMarin Pharmaceutical
- RegenxBio, Inc.
Viral Vector and Plasmid DNA Manufacturing Market Segmentation:
By Vector Type
- Adenovirus
- Retrovirus
- Adeno-Associated Virus (AAV)
- Lentivirus
- Plasmids
- Others
By Workflow
- Upstream Manufacturing
- Vector Amplification & Expansion
- Vector Recovery/Harvesting
- Downstream Manufacturing
- Purification
- Fill Finish
By Application
- Antisense & RNAi Therapy
- Gene Therapy
- Cell Therapy
- Vaccinology
- Research Applications
By End-use
- Pharmaceutical and Biopharmaceutical Companies
- Research Institutes
By Disease
- Cancer
- Genetic Disorders
- Infectious Diseases
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)
Future Outlook
The future of viral vector and plasmid DNA manufacturing appears highly promising, fueled by the accelerating adoption of personalized medicine and gene-based therapies. Continuous advancements in process optimization, automation, and regulatory harmonization are expected to enhance scalability, reduce costs, and improve global accessibility. As more therapies transition from clinical trials to commercial use, the market is poised for sustained growth, with innovations in vector design, delivery mechanisms, and manufacturing technologies continuing to redefine the landscape.
Buy this Premium Research Report@ http://visionresearchreports.com/report/checkout/41584
You can place an order or ask any questions, please feel free to contact
sales@visionresearchreports.com| +1 650-460-3308
