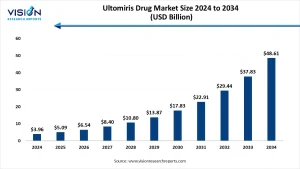
The global ultomiris drug market size was estimated at around USD 3.96 billion in 2024 and it is projected to hit around USD 48.61 billion by 2034, growing at a CAGR of 28.50% from 2025 to 2034.
Get a Sample@https://www.visionresearchreports.com/report/sample/41718
Ultomiris Drug Market Overview
Ultomiris, a long-acting complement inhibitor developed by Alexion Pharmaceuticals, is gaining significant attention as a breakthrough treatment for rare and life-threatening diseases such as paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), and generalized myasthenia gravis (gMG). As a next-generation alternative to Soliris, Ultomiris offers the benefits of less frequent dosing and improved patient compliance. With continuous research, label expansions, and regulatory approvals across regions, the drug is establishing itself as a critical component in the treatment of complement-mediated disorders.
Ultomiris Drug Market Growth
The global Ultomiris drug market is experiencing rapid growth, driven by increasing demand for targeted therapies in the rare disease space. In 2024, the market was valued at approximately USD 3.96 billion and is expected to grow exponentially to reach USD 48.61 billion by 2034, registering a CAGR of 28.50% from 2025 to 2034. This remarkable growth trajectory is fueled by ongoing clinical trials, broader reimbursement support, and increasing physician and patient awareness about complement inhibition therapies.
In addition to expanding use in existing indications, the drug’s potential in treating other autoimmune and rare disorders is attracting strong interest from healthcare providers and investors alike. The shift toward biologics and specialty drugs across healthcare systems globally further supports Ultomiris’ market expansion, making it one of the most promising assets in the biopharmaceutical pipeline.
Ultomiris Drug Market Dynamics
Driven by
- Rising incidence of complement-mediated rare diseases.
- Strong clinical efficacy and patient convenience with extended dosing intervals.
- Regulatory designations such as orphan drug status encouraging fast-track approvals.
Opportunities
- Expansion into additional therapeutic areas such as neurology and nephrology.
- Growing demand in emerging markets with improved healthcare infrastructure.
- Strategic partnerships and licensing deals to boost global distribution.
Challenges
- High cost of treatment may limit accessibility and payer acceptance.
- Competition from biosimilars and alternative therapies post-patent expiry.
- Regulatory scrutiny and post-marketing surveillance for long-term safety.
Applications of Ultomiris Drug Market
Ultomiris is primarily used to treat rare complement-mediated disorders, and its clinical applications continue to expand through research and regulatory approvals:
- Paroxysmal Nocturnal Hemoglobinuria (PNH): Reduces hemolysis and improves quality of life in patients with chronic red blood cell destruction.
- Atypical Hemolytic Uremic Syndrome (aHUS): Prevents complement-mediated thrombotic microangiopathy affecting kidneys and other organs.
- Generalized Myasthenia Gravis (gMG): Helps manage autoimmune muscle weakness in patients positive for anti-AChR antibodies.
- Neuromyelitis Optica Spectrum Disorder (NMOSD): Investigated for efficacy in reducing relapses in complement-mediated demyelinating diseases.
- Potential Future Uses: Research is ongoing for conditions like complement 3 glomerulopathy (C3G) and amyotrophic lateral sclerosis (ALS).
Ultomiris Drug Market Trends
- Increased adoption of long-acting biologics due to better dosing schedules and compliance.
- Ongoing label expansion into new rare and ultra-rare disease indications.
- Rising investments in rare disease research and orphan drug development.
- Growing competition in complement inhibition therapies with emerging biosimilars and new entrants.
Read More:https://www.heathcareinsights.com/antifungal-drugs-market/
Top Companies in Ultomiris Drug Market
- Roche Holding AG
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Johnson & Johnson
- Amgen Inc.
- Biogen Inc.
- Regeneron Pharmaceuticals, Inc.
- Takeda Pharmaceutical Company Limited
Ultomiris Drug Market Segments
By Indication
- Paroxysmal Nocturnal Hemoglobinuria (PNH)
- Atypical Hemolytic Uremic Syndrome (aHUS)
- Generalized Myasthenia Gravis (gMG)
- Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Other Rare Disorders
By Route of Administration:
- Intravenous
- Subcutaneous (in development)
- By End User:
- Hospitals
- Specialty Clinics
- Homecare Settings
By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
Future Outlook
The future of the Ultomiris market looks promising with significant growth potential over the next decade. As clinical trials validate its efficacy in new indications and more regions grant regulatory approvals, its global footprint is expected to expand substantially. With advances in biologics manufacturing and increasing support for rare disease therapies, Ultomiris is poised to remain a key player in the rare disease drug market, reshaping patient outcomes and commercial opportunities worldwide.
Buy this Premium Research Report@https://www.visionresearchreports.com/report/checkout/41719
You can place an order or ask any questions, please feel free to contact
sales@visionresearchreports.com| +1 650-460-3308
