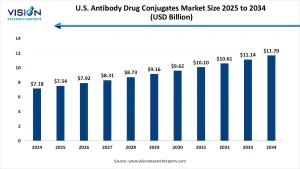
The U.S. Antibody Drug Conjugates (ADC) market was valued at USD 7.18 billion in 2024 and is expected to reach approximately USD 11.70 billion by 2034, expanding at a compound annual growth rate (CAGR) of 5% from 2025 to 2034. This growth is primarily fueled by the increasing incidence of cancer and the rising demand for targeted treatment options.
U.S. Antibody Drug Conjugates Market Overview
Antibody Drug Conjugates (ADCs) are an advanced class of biopharmaceutical drugs that combine the precision of monoclonal antibodies with the potent cell-killing ability of cytotoxic drugs. Designed to selectively target and destroy cancer cells while minimizing damage to healthy tissues, ADCs are rapidly transforming oncology treatment landscapes. The U.S. market has witnessed significant investment and innovation in this space, driven by increasing cancer prevalence and demand for precision medicine.
Get a Sample@ https://www.visionresearchreports.com/report/sample/41773
U.S. Antibody Drug Conjugates Market Growth
The U.S. antibody drug conjugates market was valued at USD 7.18 billion in 2024 and is projected to reach USD 11.70 billion by 2034, expanding at a CAGR of 5% from 2025 to 2034. This growth trajectory is fueled by rising FDA approvals, expanding clinical trials, and the successful commercialization of breakthrough ADC therapies.
Pharmaceutical companies are increasingly focusing on ADC pipeline development, supported by technological advancements in linker chemistry, payloads, and antibody engineering. As oncology remains a high-priority therapeutic area, ADCs are becoming a key component of many treatment strategies, especially for difficult-to-treat cancers.
U.S. Antibody Drug Conjugates Market Trends
- Expanding Oncology Applications: ADCs are now being developed for a broader range of cancers, including breast, lung, urothelial, and hematologic malignancies. This diversification is expanding the total addressable market.
- Rising Number of Collaborations and Licensing Deals: Partnerships between biotech firms and large pharmaceutical companies are accelerating innovation and market entry for new ADCs.
- Technological Innovation: Enhanced payloads and more stable linker technologies are improving therapeutic windows and reducing off-target toxicity.
U.S. Antibody Drug Conjugates Market Dynamics
Drivers
The U.S. antibody drug conjugates market is primarily driven by the growing incidence of cancer, which continues to be a major public health concern. As the demand for targeted and less toxic therapies rises, ADCs are emerging as a preferred treatment option. Additionally, the market benefits from a robust pipeline of ADC candidates and increasing clinical success rates, which indicate strong future growth potential. A favorable regulatory environment, including accelerated approval pathways from the FDA, further supports rapid innovation and market entry.
Opportunities
Significant opportunities exist in the development of ADCs for non-oncologic diseases, such as autoimmune and infectious conditions, which could broaden their clinical utility. Moreover, integrating ADCs with immunotherapies and other targeted treatments offers the potential for synergistic effects and improved patient outcomes. Ongoing research in personalized and precision medicine is also opening new avenues for patient-specific ADC therapies, enhancing treatment efficacy and safety.
Challenges
Despite the promising outlook, the market faces notable challenges. The high manufacturing costs and technical complexity of ADC production make scalability and affordability major concerns. There is also limited awareness among healthcare providers and patients regarding ADC therapies, which can hinder adoption. Furthermore, the risk of drug resistance and potential for adverse immune reactions remain significant clinical hurdles that researchers and manufacturers must address through innovation and safety enhancements.
What Are Antibody Drug Conjugates (ADCs)?
Antibody Drug Conjugates (ADCs) are innovative biopharmaceuticals that combine the targeting ability of monoclonal antibodies with the cancer-killing power of cytotoxic drugs. Each ADC consists of three key components: an antibody that seeks out specific antigens on cancer cells, a linker that holds the drug to the antibody, and a payload—a highly potent chemotherapy agent. Once the ADC binds to a cancer cell, it is absorbed and the payload is released inside the cell, causing targeted destruction. This precision approach aims to kill cancer cells while sparing healthy tissues, minimizing the severe side effects often associated with conventional chemotherapy.
Key Factors Fueling Market Expansion
Several critical drivers are contributing to the rapid growth of the ADC market in the U.S. First, the increasing prevalence of cancer is generating high demand for effective and less toxic therapies. ADCs offer a personalized approach, aligning with the broader trend toward precision medicine.
Secondly, regulatory support from agencies like the FDA is expediting the development and approval of ADCs through accelerated pathways and breakthrough therapy designations. This has boosted investor confidence and attracted significant funding to ADC R&D.
Lastly, pharmaceutical collaborations and strategic licensing deals are fostering innovation and accelerating commercialization. Companies are leveraging one another’s strengths in antibody engineering, drug payloads, and manufacturing expertise, making ADCs a central focus in oncology drug portfolios.
How ADCs Compare to Traditional Chemotherapy
Traditional chemotherapy involves administering cytotoxic drugs that kill rapidly dividing cells, both cancerous and healthy, leading to severe side effects such as hair loss, fatigue, nausea, and immune suppression. In contrast, ADCs are highly targeted, delivering their toxic payloads directly to cancer cells while largely sparing healthy tissues.
This targeted mechanism allows for higher efficacy with reduced systemic toxicity. ADCs can also be effective against drug-resistant cancers that no longer respond to standard treatments. While ADCs still have side effects, their safety profile is generally more manageable, making them a more tolerable and effective option for many patients.
Technological Innovations Shaping the ADC Space
- Advanced Linker Technologies: New generations of linkers are more stable in the bloodstream and release the drug only upon entering the cancer cell, increasing precision and reducing side effects.
- Next-Gen Payloads: The development of novel cytotoxic agents with improved potency and safety is enhancing ADC effectiveness, especially in drug-resistant tumors.
- Site-Specific Conjugation: This technique ensures consistent and controlled attachment of payloads to antibodies, improving efficacy and reducing variability in production.
- Target Discovery and Biomarker Integration: With the rise of genomics and bioinformatics, researchers are identifying novel cancer-specific targets, allowing for the design of ADCs tailored to individual patient profiles.
Strategic Recommendations for Stakeholders
For pharmaceutical companies, investing in platform technologies for linker and payload development is essential to stay competitive. Collaborations with academic institutions and biotech startups can accelerate innovation and reduce development timelines.
Investors should look for companies with strong ADC pipelines, demonstrated clinical success, and strategic partnerships. The ADC space is poised for sustained growth, and early-stage funding in novel platforms could yield significant returns.
Healthcare providers should invest in education and training to better understand ADC therapies, identify suitable patients, and manage associated risks.
Regulatory bodies and policymakers can continue to support this sector by maintaining clear, streamlined approval processes and encouraging public-private partnerships to expand access and affordability.
Applications in the U.S. Antibody Drug Conjugates Market
Antibody Drug Conjugates (ADCs) are revolutionizing cancer treatment by offering targeted therapy with reduced side effects. One of the most successful applications has been in treating HER2-positive breast cancer, where ADCs like Enhertu have significantly improved patient outcomes. Additionally, ADCs are being used in the management of relapsed or refractory multiple myeloma, providing a new option for patients who have exhausted traditional therapies. In the area of lung cancer, particularly in late-stage and metastatic cases, ADCs are showing strong potential to enhance survival rates and quality of life. Moreover, emerging clinical trials highlight the promise of ADCs in treating ovarian and gastric cancers, indicating a broader therapeutic scope beyond currently approved indications.
Case Study Enhertu (Trastuzumab Deruxtecan)
Enhertu, developed by AstraZeneca and Daiichi Sankyo, has emerged as a leading ADC for HER2-positive breast and gastric cancers. Its success showcases the potential of next-generation ADCs with superior efficacy and safety profiles. With FDA Breakthrough Therapy Designation and accelerated approval, Enhertu demonstrates how advanced ADCs are reshaping treatment paradigms and becoming blockbusters in oncology.
Read More:https://www.heathcareinsights.com/athleisure-market/
Top Companies in U.S. Antibody Drug Conjugates Market
- Takeda Pharmaceutical Company Ltd.
- AstraZeneca Plc
- F. Hoffmann-La Roche Ltd.
- Pfizer, Inc.
- Gilead Sciences, Inc.
- Daiichi Sankyo Company Ltd.
- GlaxoSmithKline Plc
- Astellas Pharma, Inc.
- ADC Therapeutics SA
Want custom data? Click here: https://www.visionresearchreports.com/report/customization/41773
U.S. Antibody Drug Conjugates Market Segmentation
By Application
- Blood Cancer
- Leukemia
- Lymphoma
- Multiple Myeloma
- Breast Cancer
- Urothelial Cancer & Bladder Cancer
- Other Cancer
By Product
- Kadcyla
- Enhertu
- Adcetris
- Padcev
- Trodelvy
- Polivy
- Others
By Target
- HER2
- CD22
- CD30
- Others
By Technology
- Type
- Cleavable Linker
- Non-cleavable Linker
- Linkerless
- Linker Technology Type
- VC
- Sulfo-SPDB
- VA
- Hydrazone
- Others
- Payload Technology
- MMAE
- MMAF
- DM4
- Camptothecin
- Others
Future Outlook
The U.S. ADC market is expected to evolve with the integration of novel biomarkers, artificial intelligence in drug discovery, and companion diagnostics. As ADCs become more refined and accessible, their application may expand beyond cancer into autoimmune diseases and infectious conditions. With increasing investment and public-private partnerships, the market will likely witness a surge in innovation and patient access in the next decade.
Buy this Premium Research Report@https://www.visionresearchreports.com/report/checkout/41773
You can place an order or ask any questions, please feel free to contact
sales@visionresearchreports.com| +1 650-460-3308
