The U.S. active pharmaceutical ingredients market size was valued at USD 87.75 billion in 2024 and is projected to surpass around USD 141.58 billion by 2033, registering a CAGR of 4.9% over the forecast period of 2025 to 2034.
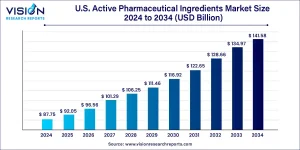
U.S. Active Pharmaceutical Ingredients Market Overview
The U.S. Active Pharmaceutical Ingredients (API) market plays a critical role in the pharmaceutical value chain, providing the essential components that give medications their therapeutic effects. APIs are the biologically active elements in drugs, and their quality and reliability are crucial for the safety and efficacy of pharmaceutical products. As the U.S. continues to lead in pharmaceutical innovation and production, the API market remains a backbone for both branded and generic drug development.
Get a Sample@https://www.visionresearchreports.com/report/sample/41637
U.S. Active Pharmaceutical Ingredients Market Growth
The rising prevalence of chronic diseases such as cancer, cardiovascular conditions, and diabetes is significantly driving the demand for APIs in the United States. With an aging population and increasing health awareness, there is a strong need for high-quality and cost-effective drug ingredients that can support long-term treatment plans. Moreover, personalized medicine and targeted therapies are gaining traction, requiring sophisticated APIs with specific molecular compositions.
Another factor contributing to growth is the expanding biopharmaceutical sector. As biologic drugs gain prominence over traditional small-molecule drugs, the demand for complex and high-potency APIs (HPAPIs) is accelerating. Additionally, the COVID-19 pandemic has reinforced the importance of a resilient domestic API supply chain, encouraging investment in U.S.-based production facilities and contract manufacturing organizations (CMOs).
U.S. Active Pharmaceutical Ingredients Market Trends
- Rising Outsourcing to CDMOs: Pharmaceutical companies are increasingly outsourcing API production to Contract Development and Manufacturing Organizations (CDMOs) to reduce costs and enhance scalability.
- Shift Toward Biotech APIs: There is a growing preference for biotech-derived APIs such as monoclonal antibodies and recombinant proteins due to the surge in biologic drug development.
- Growing Use of Green Chemistry: Manufacturers are adopting sustainable practices and green chemistry techniques to reduce environmental impact and comply with regulatory standards.
- Technological Advancements in API Manufacturing: The adoption of continuous manufacturing, automation, and real-time monitoring systems is improving production efficiency and quality assurance.
Types of APIs and How They’re Made
APIs can be classified into two main types
- Synthetic APIs: These are chemically produced using traditional methods in labs. They’re common in small-molecule drugs such as painkillers, antibiotics, and antihistamines. Production involves multiple chemical reactions, purification, and crystallization stages to ensure purity and potency.
- Biotech APIs (Biological APIs): These are derived from living organisms using biotechnology processes such as fermentation or cell culture. They’re used in complex drugs like insulin, monoclonal antibodies, and vaccines. Biotech API manufacturing is highly sensitive and requires advanced bioreactors and sterile environments.
Manufacturing APIs involves several phases including research and development, process design, quality control, and regulatory compliance. Each API must meet strict standards set by the FDA and other authorities.
Where APIs Are Used in Healthcare?
APIs form the active component in all modern medicines. Some key applications include
- Chronic Disease Treatment: Used in drugs for diabetes, hypertension, cancer, and heart disease.
- Infectious Disease Control: Key ingredient in antivirals, antibiotics, and antifungals.
- Pain Management: Found in opioids and non-opioid analgesics.
- Mental Health Medications: Essential in antidepressants, antipsychotics, and mood stabilizers.
- Specialty and Rare Disease Drugs: Used in orphan drugs and biologics for niche medical needs.
APIs are also critical in veterinary medicine, clinical trials, and personalized treatments, making them foundational to healthcare innovation.
Big Opportunities in API Manufacturing
The U.S. API market is full of growth opportunities, especially due to:
- Reshoring of Production: There’s increasing government and industry focus on reducing dependence on foreign API suppliers, especially from Asia. This opens doors for domestic manufacturers.
- Expansion of Biologics and Biosimilars: As more biologic drugs enter the market, the need for biotech APIs is surging, requiring advanced facilities and expertise.
- Investment in Green Chemistry: Sustainable, eco-friendly API manufacturing methods are gaining support, creating opportunities for innovation in process design.
- Contract Manufacturing Growth: Pharmaceutical companies are outsourcing API production to CDMOs (Contract Development and Manufacturing Organizations), enabling small and mid-sized manufacturers to enter the market.
Latest Trends Shaping the API Industry
- Increased Use of Continuous Manufacturing: Moving away from batch production, continuous manufacturing offers better efficiency, consistent quality, and scalability.
- Focus on High-Potency APIs (HPAPIs): With the rise of oncology and targeted drugs, there is growing demand for HPAPIs that require specialized handling and containment technologies.
- Adoption of Digital and AI Tools: Artificial intelligence and machine learning are being used to optimize API synthesis routes, predict yield improvements, and ensure regulatory compliance.
U.S. Active Pharmaceutical Ingredients Market Dynamics
Drivers
The U.S. API market is primarily driven by the increased prevalence of lifestyle-related and chronic diseases, such as diabetes, cancer, and cardiovascular disorders. This growing disease burden fuels consistent demand for effective pharmaceutical treatments. Additionally, the rising demand for both generic and branded drugs is accelerating API consumption across the pharmaceutical sector. Government support through policy reforms and funding for domestic API production is also playing a vital role in reducing reliance on international suppliers. Moreover, the growth of specialty and high-potency APIs (HPAPIs) is opening new avenues for advanced therapeutics, especially in oncology and rare diseases.
Opportunities
The market presents significant opportunities with the expansion of biologics and biosimilars, as these complex drugs require sophisticated API development processes. Increasing investment in artificial intelligence (AI) and digital tools is enhancing drug discovery and API manufacturing efficiency. Furthermore, strategic partnerships between pharmaceutical and technology companies are helping accelerate innovation, streamline production, and improve overall drug quality and delivery.
Challenges
Despite its growth potential, the API market faces several challenges. Stringent regulatory requirements for safety, quality, and environmental compliance often lead to lengthy approval processes. The high costs associated with API development and setting up compliant manufacturing infrastructure pose financial barriers, particularly for smaller firms. Additionally, supply chain vulnerabilities, especially for critical raw materials and intermediates sourced globally, can disrupt production timelines and increase dependency on imports.
Applications in the U.S. Active Pharmaceutical Ingredients Market
Active Pharmaceutical Ingredients (APIs) have a wide range of applications across the healthcare and life sciences sectors. One of their primary uses is in pharmaceutical manufacturing, where they serve as the essential components in both generic and branded drug formulations. APIs determine the therapeutic effect of medications, making their quality and consistency critical.
In clinical research, APIs play a vital role during drug development and trials. Researchers use them to evaluate the safety, efficacy, and dosage of new medications in controlled environments. The rise of personalized medicine has further expanded API applications, with custom-designed APIs being developed for individualized treatments based on a patient’s genetic profile or specific condition.
Case Study Domestic API Manufacturing Boost
In response to supply chain disruptions during the COVID-19 pandemic, a major U.S. pharmaceutical company partnered with a domestic CMO to establish a new API production facility in North Carolina. This project, supported by government funding, focused on producing critical ingredients for antibiotics and antivirals. As a result, the company achieved supply chain independence for key drugs and reduced lead times by 40%. This initiative also contributed to local job creation and set a benchmark for API reshoring strategies across the industry.
Read More:https://www.heathcareinsights.com/trauma-care-centers-market/
U.S. Active Pharmaceutical Ingredients Market Top Key Companies
- AbbVie Inc.
- Viatris Inc.
- Fresenius Kabi AG.
- Curia.
- Pfizer Inc. (Pfizer Center One)
- Bristol-Myers Squibb Company
- Catalent, Inc.
- Ampac Fine Chemicals (AFC)
- Amgen Inc.
- Johnson & Johnson
Want custom data? Click here:https://www.visionresearchreports.com/report/sample/41637
U.S. Active Pharmaceutical Ingredients Market Segmentation
By Types of Synthesis
- Synthetic
- Biotech
By Types of Manufacturers
- Captive APIs
- Merchant APIs
By Types
- Innovative APIs
- Generic APIs
By Application
- Cardiovascular Diseases
- Endocrinology
- CNS and Neurology
- Oncology
- Gastroenterology
- Orthopedic
- Pulmonology
- Nephrology
- Ophthalmology
- Others
Future Outlook
The future of the U.S. API market is closely tied to technological innovation, regulatory evolution, and the ongoing biopharmaceutical boom. Over the next decade, advancements in areas such as synthetic biology, continuous manufacturing, and AI-driven drug development are expected to transform how APIs are designed and produced. These technologies will enable more efficient, cost-effective, and scalable manufacturing processes, ensuring faster time-to-market for new therapies.
Additionally, the shift toward personalized and precision medicine will require highly specialized APIs tailored to individual patient needs, further driving innovation in formulation and production. Government efforts to strengthen domestic supply chains and reduce dependency on foreign sources will also create opportunities for U.S.-based API manufacturers. As biologics, biosimilars, and specialty drugs become more prevalent, the demand for high-quality, high-potency APIs will continue to rise positioning the U.S. as a global leader in advanced pharmaceutical ingredient production.
Buy this Premium Research Report@https://www.visionresearchreports.com/report/checkout/41637
You can place an order or ask any questions, please feel free to contact
sales@visionresearchreports.com| +1 650-460-3308
