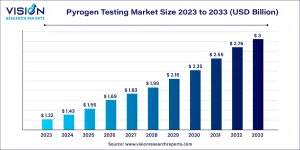
The global pyrogen testing market was valued at approximately USD 1.32 billion in 2023 and is expected to reach nearly USD 3 billion by 2033, expanding at a CAGR of 8.56% between 2024 and 2033.
The pyrogen testing market is driven by the rapid growth of pharmaceutical and biotechnology R&D, the rising demand for biologics and biosimilars, and increasing concerns over drug safety and contamination. Strict regulatory mandates, combined with a global shift toward animal-free and ethical testing methods, are further accelerating the adoption of advanced in-vitro assays and automation technologies.
Introduction to Pyrogen Testing
Pyrogen testing is a crucial process in the pharmaceutical and biotechnology industries that ensures medical products are free from harmful fever-causing substances. Pyrogens, which include bacterial endotoxins and other microbial contaminants, can trigger severe immune responses when introduced into the human body through injectables, biologics, or medical devices. To prevent such risks, pyrogen testing acts as a safeguard for patient safety and product quality. Over time, testing methods have advanced from animal-based techniques to innovative, ethical, and faster in-vitro alternatives.
Pyrogen Testing Market Overview
The pyrogen testing market plays a crucial role in the global healthcare and pharmaceutical ecosystem, ensuring that drugs, vaccines, medical devices, and biologics are free from harmful pyrogenic contaminants. Pyrogens are fever-inducing substances that can cause severe adverse reactions if they enter the bloodstream through injectable therapies or implants. With the rapid expansion of the biopharmaceutical sector, advancements in biologics, and rising regulatory scrutiny, demand for pyrogen testing has significantly grown. The industry has evolved from traditional rabbit tests to highly sophisticated in-vitro assays that prioritize accuracy, efficiency, and ethical compliance.
Access Your Sample Report Today: https://www.visionresearchreports.com/report/sample/41304
Pyrogen Testing Market Growth
The market has witnessed steady expansion due to the rising incidence of chronic diseases, increasing production of injectable drugs, and heightened focus on patient safety. Biologics and biosimilars, being more sensitive to contamination risks, have further fueled the adoption of modern pyrogen testing methods. Pharmaceutical and biotechnology companies are investing heavily in advanced assays that provide quicker and more reliable results while aligning with the global shift toward animal-free testing models.
In addition, supportive regulations across the U.S., Europe, and Asia are encouraging the adoption of innovative testing platforms. The acceleration of vaccine development, especially in response to global health emergencies, has underscored the critical role of pyrogen testing in reducing risks and ensuring quality. This trend is expected to sustain strong growth momentum over the coming years.
Why Pyrogen Testing Matters
The importance of pyrogen testing lies in its ability to protect human health and maintain trust in healthcare systems. Even the smallest contamination in an injectable drug or medical device can lead to fever, shock, or life-threatening conditions. For pharmaceutical manufacturers, regulatory compliance makes pyrogen testing a mandatory step before products reach patients. Beyond safety, these tests also enhance efficiency in production, as early detection of contaminants helps reduce costly recalls and delays. Simply put, pyrogen testing is the backbone of quality assurance in modern medicine.
Pyrogen Testing Market Trends
- Shift toward animal-free testing methods: There is a growing transition from rabbit pyrogen tests to recombinant factor C (rFC) assays and monocyte activation tests (MAT), driven by ethical concerns and regulatory approval.
- Integration of automation and high-throughput screening: Automated systems are being adopted to enhance efficiency, reduce human error, and enable large-scale testing in pharmaceutical manufacturing facilities.
- Focus on regulatory harmonization: Global agencies are aligning testing standards to ensure consistency, which is boosting cross-border trade of pharmaceuticals and medical devices.
- Rising demand in biologics and personalized medicine: As biologics become more central in treatments for cancer, autoimmune diseases, and rare disorders, the need for precise pyrogen testing has intensified.
Why Pyrogen Testing Matters
The importance of pyrogen testing lies in its ability to protect human health and maintain trust in healthcare systems. Even the smallest contamination in an injectable drug or medical device can lead to fever, shock, or life-threatening conditions. For pharmaceutical manufacturers, regulatory compliance makes pyrogen testing a mandatory step before products reach patients. Beyond safety, these tests also enhance efficiency in production, as early detection of contaminants helps reduce costly recalls and delays. Simply put, pyrogen testing is the backbone of quality assurance in modern medicine.
Pyrogen Testing Market Dynamics
Drivers
The pyrogen testing market is primarily driven by the rapid growth of pharmaceutical and biotechnology research and development (R&D) activities. With companies constantly working on new drug formulations, vaccines, and advanced biologics, the need for rigorous testing to ensure product safety has become indispensable. This surge in R&D spending continues to fuel demand for reliable pyrogen detection methods.
Another significant driver is the expanding biologics and biosimilars market, which requires more sensitive testing due to the complex structures of these drugs. Biologics are highly prone to contamination, and even minimal traces of pyrogens can cause adverse reactions. Hence, the increasing adoption of biologics across therapeutic areas is amplifying the importance of advanced pyrogen testing technologies.
Opportunities
A key opportunity lies in the adoption of in-vitro testing alternatives, which present vast potential for innovation and growth. Recombinant factor C assays and monocyte activation tests are gaining popularity as ethical, reliable, and faster alternatives to animal-based testing. This shift opens avenues for companies developing novel assay kits and instruments.
Emerging economies such as India, China, and Brazil offer untapped growth potential. The increasing number of pharmaceutical manufacturing plants in these regions, coupled with government initiatives to strengthen healthcare infrastructure, is expected to create a lucrative market for pyrogen testing providers.
Challenges
Despite strong growth prospects, the market faces certain hurdles. High costs associated with advanced pyrogen testing systems remain a barrier, especially for small and mid-sized pharmaceutical manufacturers. The initial investment in equipment and consumables can discourage companies with limited budgets from transitioning to modern testing solutions.
Another challenge is the lack of awareness among smaller companies about the benefits and regulatory acceptance of animal-free alternatives. Many continue to rely on traditional rabbit pyrogen tests or Limulus Amebocyte Lysate (LAL) methods due to familiarity, even when better options exist. This resistance slows down the widespread adoption of innovative assays.
Lastly, technical limitations in detecting all types of pyrogens using a single method create complexities for manufacturers. For instance, while rFC assays are highly effective for endotoxin detection, they may not cover all non-endotoxin pyrogens. This limitation compels companies to use multiple tests, adding to costs and operational inefficiencies.
Want custom data? Click here: https://www.visionresearchreports.com/report/customization/41304
Applications in the Market
Pyrogen testing serves as a cornerstone in ensuring product safety across multiple industries. In the pharmaceutical sector, it is widely applied for the quality control of injectable drugs, vaccines, and intravenous therapies, where the presence of pyrogens can cause severe adverse reactions in patients. With the rapid pace of vaccine development, particularly during public health emergencies such as pandemics, pyrogen testing has become more critical than ever to ensure product reliability and regulatory compliance.
In the biotechnology industry, pyrogen testing plays a vital role in the development and manufacturing of biologics, biosimilars, and monoclonal antibodies. These products are highly complex in nature and more vulnerable to contamination, making rigorous testing essential for maintaining therapeutic effectiveness and patient safety.
The medical device sector also relies heavily on pyrogen testing to ensure that implants, surgical instruments, and other invasive devices are free from harmful contaminants. Testing ensures that these products do not trigger unwanted immune responses or complications after coming into contact with the human body. This is particularly important for devices like catheters, dialysis equipment, and cardiovascular implants.
Pyrogen Testing Market Regional Analysis
- North America
North America continues to hold the largest share of the pyrogen testing market, primarily due to its strong pharmaceutical and biotechnology manufacturing base, coupled with advanced healthcare infrastructure. The region benefits from well-established regulatory frameworks led by agencies such as the U.S. FDA, which mandate strict compliance for injectable drugs, vaccines, and medical devices. Moreover, the presence of global industry leaders, early adoption of cutting-edge technologies, and significant investments in R&D further reinforce the region’s dominance. Increasing focus on biologics and personalized medicine in the U.S. and Canada is expected to sustain market leadership in the coming years.
- Europe
Europe ranks as the second-largest market, driven by rapid adoption of animal-free testing methods such as recombinant factor C assays and monocyte activation tests. The European Medicines Agency (EMA) and the European Pharmacopoeia have been at the forefront of pushing for animal welfare in testing, encouraging companies to transition toward in-vitro alternatives. Additionally, strong government support, a collaborative research ecosystem, and rising demand for biosimilars across Germany, the U.K., and France are further propelling growth in the region. Europe is also emerging as a hub for innovations in sustainable and ethical laboratory practices.
- Asia-Pacific
Asia-Pacific is poised to be the fastest-growing market, with countries like India, China, Japan, and South Korea establishing themselves as global pharmaceutical and biotechnology hubs. These nations are witnessing substantial investments in manufacturing capabilities, vaccine development, and biologics production, all of which require rigorous pyrogen testing protocols. Rising government initiatives to strengthen regulatory compliance, coupled with a large patient base and lower production costs, are making the region highly attractive for international players. Moreover, growing collaborations between local manufacturers and global companies are accelerating the adoption of advanced pyrogen testing technologies.
- Latin America
Latin America presents a growing market, supported by increasing pharmaceutical manufacturing activity in countries such as Brazil and Mexico. Although regulatory frameworks are still evolving, governments are taking steps to align with international standards, boosting awareness of drug safety and contamination risks. The region is gradually adopting modern testing methods as part of its efforts to improve healthcare outcomes and attract international pharmaceutical investment.
- Middle East & Africa (MEA)
Middle East & Africa (MEA) remains at a nascent stage but holds long-term potential. The rising prevalence of chronic diseases, expansion of healthcare facilities, and government-driven healthcare reforms are contributing to increased demand for safe and effective drugs. Countries like the UAE and Saudi Arabia are investing in building advanced pharmaceutical infrastructure, which will support the adoption of pyrogen testing solutions. While challenges such as limited skilled workforce and high costs of advanced systems persist, ongoing initiatives to modernize healthcare are expected to create growth opportunities over the next decade.
Case Study: Transition to Modern Pyrogen Testing for Enhanced Compliance and Efficiency
A leading European pharmaceutical company faced increasing challenges with its reliance on the traditional rabbit pyrogen test (RPT). While effective, the method was time-consuming, costly, and increasingly scrutinized under the European Union’s stringent animal welfare directives. With the growing demand for biologics and injectable therapies, the company recognized the need to modernize its pyrogen testing approach to maintain regulatory compliance and operational efficiency.
To address these challenges, the company transitioned to the recombinant factor C (rFC) assay, an advanced in-vitro alternative designed to detect endotoxins without animal use. By implementing this technology, the company reduced testing timelines significantly, enabling faster product release cycles. This transition not only aligned with EU guidelines advocating animal-free testing but also positioned the company as a leader in adopting sustainable and ethical practices.
Read More: https://www.heathcareinsights.com/u-s-point-of-care-glucose-testing-market/
Pyrogen Testing Market Key Players
- Charles River Laboratories
- Ellab A/S
- Merck KGaA
- GenScript Biotech
- bioMérieux SA
- Lonza Group
- Thermo Fisher Scientific, Inc.
- Associates of Cape Cod, Inc.
- FUJIFILM Wako Chemicals U.S.A. Corporation (Pyrostar)
Pyrogen Testing Market Segmentation
By Product
- Consumables
- Instruments
- Services
By Test Type
- LAL test
- Chromogenic test
- Turbidimetric test
- Gel clot test
- In vitro pyrogen test
- Rabbit test
By End-use
- Pharmaceutical and biotechnology companies
- Medical devices companies
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa (MEA)
Future Outlook
The pyrogen testing market is set to experience sustained and accelerated growth in the coming years, fueled by the dual forces of regulatory support and technological innovation. As global health authorities increasingly promote the adoption of animal-free testing models, traditional methods such as rabbit pyrogen tests are expected to gradually phase out. This shift will not only reduce ethical concerns but also drive efficiency and reproducibility across pharmaceutical quality control systems.
One of the most promising aspects of the market’s future is the integration of advanced technologies such as artificial intelligence (AI), machine learning, and automation. These innovations will enable laboratories to process larger volumes of data more accurately, reduce human error, and enhance predictive capabilities in identifying contamination risks. Automated in-vitro platforms combined with AI-driven analytics are likely to revolutionize end-to-end pyrogen detection, ensuring faster decision-making and improved regulatory compliance.
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/checkout/41304
You can place an order or ask any questions, please feel free to contact
sales@visionresearchreports.com| +1 650-460-3308
