The global pharmaceutical CDMO market was accounted t USD 184.95 billion in 2024 and is expected to reach approximately USD 374.16 billion by 2034, expanding at a CAGR of 7.3% from 2025 to 2034. This growth is primarily fueled by the rising need for cost-efficient solutions in drug development and manufacturing.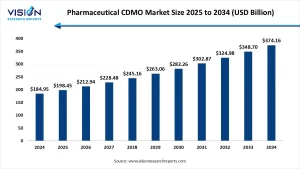
Pharmaceutical CDMO Market Overview
The pharmaceutical Contract Development and Manufacturing Organization (CDMO) market plays a vital role in supporting drug manufacturers by offering services from drug development to commercial manufacturing. These organizations have become integral to pharmaceutical operations due to their ability to reduce costs, improve scalability, and accelerate time-to-market. As the complexity of drug formulations increases, CDMOs offer critical technical expertise and advanced manufacturing infrastructure that pharmaceutical companies often lack in-house.
Get a Sample@https://www.visionresearchreports.com/report/sample/40281
Pharmaceutical CDMO Market Growth
The pharmaceutical CDMO market is experiencing substantial growth driven by increasing demand for outsourcing across the pharmaceutical value chain. Small and medium-sized pharmaceutical companies are particularly reliant on CDMOs to meet rising drug development needs without the overhead of manufacturing facilities or R&D infrastructure. Furthermore, large pharma companies are leveraging CDMO partnerships to boost operational efficiency and focus on core competencies like marketing and drug discovery.
Biologics and advanced therapies are also fueling market expansion. With complex production requirements, these therapies demand specialized expertise and infrastructure that CDMOs are well-equipped to provide. As personalized medicine and cell and gene therapies grow, CDMOs will continue to be strategic partners in delivering precision-based treatments.
Pharmaceutical CDMO Market Trends
- Increased Biologics Outsourcing: CDMOs are investing in biomanufacturing capabilities to support the rising demand for monoclonal antibodies, vaccines, and cell and gene therapies.
- Emergence of Integrated Service Models: End-to-end services from discovery to commercialization are gaining traction as pharmaceutical firms seek single-source outsourcing partners.
- Digital Transformation in Manufacturing: The adoption of Industry 4.0 technologies such as AI, IoT, and automation is improving production efficiency and traceability.
- Growth in High-Potency Active Pharmaceutical Ingredients (HPAPIs): CDMOs with containment capabilities are experiencing higher demand due to the increasing number of oncology and specialty drugs requiring HPAPI handling.
What is a CDMO and Why It Matters in Pharma
A Contract Development and Manufacturing Organization (CDMO) is a third-party company that pharmaceutical and biotech firms hire to help with the development and manufacturing of drugs. CDMOs offer a wide range of services, including drug formulation, clinical trial material production, analytical testing, commercial manufacturing, and packaging.
They are essential to the pharmaceutical industry because they allow companies—especially startups and mid-sized firms—to bring products to market without investing in costly infrastructure. This not only reduces operational costs but also speeds up drug development, increases flexibility, and ensures compliance with regulatory standards.
How CDMOs Help Make New Medicines Faster
CDMOs accelerate the drug development process by providing expertise, equipment, and ready-to-use facilities that many pharmaceutical companies may not possess internally. From early-stage development to commercial manufacturing, CDMOs manage multiple stages of the drug lifecycle, allowing pharma companies to focus on innovation and R&D.
By offering integrated services under one roof such as formulation, testing, scaling, and packaging CDMOs minimize delays caused by multiple handovers between different vendors. Their streamlined processes and experienced teams help fast-track everything from lab discovery to patient-ready products, significantly reducing time-to-market.
How CDMOs Ensure Drug Safety and Quality
Ensuring drug safety and quality is one of the top priorities for CDMOs. They follow Good Manufacturing Practices (GMP) and comply with strict regulatory guidelines set by agencies like the FDA, EMA, and WHO. CDMOs conduct rigorous testing during and after production, ensuring that each batch of a drug meets safety, purity, and efficacy standards.
Additionally, advanced quality assurance systems, automated monitoring tools, and validated processes help CDMOs detect any deviations early and correct them promptly. Their deep understanding of regulatory requirements allows for the smooth approval of products in different international markets, minimizing the risk of compliance issues.
Pharmaceutical CDMO Market Dynamics
Drivers
- Rising complexity in drug development and formulation
- Growing demand for biologics and personalized therapies
- Pharmaceutical companies focusing on core R&D and outsourcing manufacturing
Opportunities
- Expansion of CDMO services in emerging markets
- Increased investments in continuous manufacturing and modular facilities
- Partnerships in orphan drug and rare disease space
Challenges
- Capacity constraints and scalability issues for high-demand therapies
- Regulatory complexity across regions
- Quality control and IP protection risks in outsourcing relationships
Applications in the Pharmaceutical CDMO Market
Pharmaceutical CDMOs play a vital role across various stages of the drug development and manufacturing lifecycle. One of their key applications is in clinical trial material manufacturing, where they offer formulation and packaging support for investigational drugs used during clinical studies. This ensures that trial drugs are consistently produced and packaged to meet regulatory and quality standards.
In commercial drug manufacturing, CDMOs provide large-scale production capabilities for both branded and generic drugs after regulatory approval. They also contribute significantly to formulation development, helping pharmaceutical companies design effective dosage forms such as tablets, injectables, and extended-release systems tailored to specific therapeutic needs.
Case Study Lonza and Moderna Strategic Partnership
In a landmark collaboration, Moderna partnered with Lonza to scale up the production of its COVID-19 mRNA vaccine. The CDMO offered specialized mRNA manufacturing capabilities and helped Moderna ramp up its global supply. This partnership showcased the critical role CDMOs can play in rapid response manufacturing and emphasized the value of scalable, flexible production capacity.
Read More:https://www.heathcareinsights.com/veterinary-pharmacovigilance-market/
Top Companies in Pharmaceutical CDMO Market
- Lonza
- Thermo Fisher Scientific, Inc.
- Recipharm AB
- Laboratory Corporation of America Holdings (LabCorp)
- Catalent, Inc.
- WuXi AppTec, Inc.
- Samsung Biologics
- Piramal Pharma Solutions
- Siegfried Holding AG
- CordenPharma International
- Cambrex Corporation
- Bushu Pharmaceuticals Ltd.
- Nipro Corporation
Want custom data? Click here: https://www.visionresearchreports.com/report/customization/40281
Market Segmentations
By Product
- API
- By Synthetic
- Solid
- Liquid
By Type
- Traditional Active Pharmaceutical Ingredient (Traditional API)
- Highly Potent Active Pharmaceutical Ingredient (HP-API)
- Antibody Drug Conjugate (ADC)
- Others
By Drug
- Innovative
- Generics
By Manufacturing
- Continuous manufacturing
- Batch manufacturing
- Biotech
- Drug Product
- Oral Solid Dose
- Semi-solid dose
- Liquid Dose
- Others
By Workflow
- Clinical
- Commercial
By Application
- Oncology
- Hormonal
- Glaucoma
- Cardiovascular Disease
- Diabetes
- Others
By Regional
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Italy
- Spain
- Asia Pacific
- Japan
- China
- India
- Australia
- South Korea
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Future Outlook
The future of the pharmaceutical CDMO market is promising, driven by technological advancements and the growing demand for specialized therapies. The market is expected to evolve from traditional manufacturing services to innovation-driven partnerships. AI-enabled drug development, continuous manufacturing, and sustainable practices are expected to redefine how CDMOs operate. As more drugs become niche, CDMOs will need to remain agile, modular, and highly compliant with evolving regulations.
Buy this Premium Research Report@https://www.visionresearchreports.com/report/checkout/40281
You can place an order or ask any questions, please feel free to contact
sales@visionresearchreports.com| +1 650-460-3308
