The global lysosomal storage disease treatment market size was accounted at around USD 4.58 billion in 2024 and it is projected to hit around USD 7.18 billion by 2034, growing at a CAGR of 4.60% from 2025 to 2034.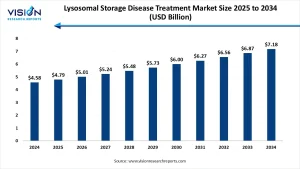
Get a Sample@https://www.visionresearchreports.com/report/sample/41753
Lysosomal Storage Disease Treatment Market Overview
The global lysosomal storage disease (LSD) treatment market is witnessing steady growth due to the rising prevalence of rare genetic disorders and the advancement of biotechnological research. In 2024, the market was valued at approximately USD 4.58 billion and is anticipated to grow to USD 7.18 billion by 2034, reflecting a CAGR of 4.60% from 2025 to 2034. LSDs are inherited metabolic disorders caused by enzyme deficiencies, and their treatment often involves enzyme replacement therapies, gene therapies, and supportive care. With growing patient awareness and improved diagnostic technologies, early detection and management of these diseases are becoming more accessible.
Lysosomal Storage Disease Treatment Market Growth
The rise in newborn screening programs and increased government initiatives supporting rare disease treatment have significantly contributed to the expansion of the LSD treatment market. Pharmaceutical companies are focusing on developing targeted therapies, including recombinant enzymes and small molecules, tailored for individual disorders such as Gaucher disease, Fabry disease, and Pompe disease. These innovations have improved the quality of life for patients and have driven the commercial interest in rare disease therapies.
Additionally, regulatory support in the form of orphan drug designations and fast-track approvals is encouraging drug development. Market players are investing heavily in research and collaborations to expand their treatment pipelines. This proactive approach is expected to sustain the market’s growth over the next decade.
How LSD Treatments Are Applied in Real-World Settings
In real-world clinical settings, lysosomal storage disease (LSD) treatments are carefully tailored to each patient’s specific condition, disease stage, and genetic profile. The most commonly used treatment approach is Enzyme Replacement Therapy (ERT), where the missing or deficient enzyme is administered intravenously, typically in a hospital or infusion center. ERT has shown significant success in conditions such as Gaucher disease, Fabry disease, and Pompe disease by helping manage symptoms and slow disease progression.
For patients in remote or stable conditions, home-based infusion services are increasingly being adopted, improving treatment adherence and convenience. In pediatric cases, early diagnosis through newborn screening enables clinicians to initiate therapy before irreversible damage occurs, greatly enhancing outcomes. Additionally, Substrate Reduction Therapies (SRTs) and supportive medications are used alongside ERT to manage symptoms such as pain, fatigue, or organ complications.
Lysosomal Storage Disease Treatment Market Dynamics
Drivers
- Increasing prevalence of lysosomal storage diseases worldwide.
- Advancements in biotechnology and genetic research.
- Supportive government policies and orphan drug status incentives.
Opportunities
- Emerging markets with growing healthcare infrastructure.
- Development of innovative gene and stem cell therapies.
- Expansion of newborn screening programs globally.
Challenges
- High cost of treatment and limited access in low-income regions.
- Limited awareness and diagnostic capabilities in developing countries.
- Small patient populations affecting commercial viability.
Impact of COVID-19 on the Lysosomal Storage Disease Treatment Market
The COVID-19 pandemic had a mixed impact on the lysosomal storage disease (LSD) treatment market, affecting both patients and healthcare providers.
In the early stages of the pandemic, disruptions in healthcare services and supply chains led to delays in diagnosis and interruptions in treatment schedules, particularly for patients dependent on in-hospital enzyme replacement therapies. Travel restrictions and limited access to infusion centers meant that many patients missed or postponed their regular treatments, potentially worsening disease outcomes.
However, the crisis also accelerated the adoption of home-based infusion services and telemedicine, which provided more flexible care options for LSD patients. Pharmaceutical companies and healthcare providers responded by implementing remote monitoring and support systems, ensuring continuity of care while reducing the risk of virus exposure.
Applications in the Market
Lysosomal storage disease treatments are primarily used in hospitals and specialty clinics for managing chronic symptoms and preventing disease progression. These therapies are crucial in pediatric care, where early treatment can significantly reduce developmental delays. In research settings, experimental therapies are being developed to replace faulty genes or correct enzyme deficiencies at the molecular level.
Real-World Impact – A Case Study
Case: Gaucher Disease Management through ERT
A 12-year-old patient diagnosed with Gaucher disease in the United States began enzyme replacement therapy shortly after diagnosis. Over the span of 18 months, the patient exhibited marked improvements in spleen and liver size, reduced bone pain, and normalized hemoglobin levels. The success of this case highlights the importance of early diagnosis and sustained treatment adherence, as well as the potential of ERT to significantly improve life expectancy and quality.
Lysosomal Storage Disease Treatment Market Trends
- Shift Towards Gene Therapy: Advanced gene therapy techniques are emerging as promising alternatives to traditional enzyme replacement therapies, with several candidates in clinical trials.
- Focus on Personalized Medicine: Precision medicine approaches are being adopted to customize treatment plans based on patient-specific genetic profiles, enhancing efficacy and reducing side effects.
- Increased FDA Approvals for Rare Disease Drugs: A higher number of drugs targeting rare lysosomal disorders have received regulatory approvals in recent years, boosting patient access to treatment.
- Growing Collaborations and Licensing Deals: Strategic partnerships between biotech firms and pharmaceutical companies are facilitating knowledge sharing and accelerating treatment innovation.
Read More:https://www.heathcareinsights.com/diabetic-ketoacidosis-treatment-market/
Top Companies in Lysosomal Storage Disease Treatment Market
- Takeda Pharmaceutical Company Limited
- BioMarin Pharmaceutical Inc.
- Amicus Therapeutics, Inc.
- Pfizer Inc.
- Johnson & Johnson
- Alexion Pharmaceuticals, Inc. (AstraZeneca)
- Avrobio, Inc.
- Actelion Pharmaceuticals Ltd
- Protalix BioTherapeutics, Inc.
Market Segments
By Treatment Type
- Enzyme Replacement Therapy (ERT)
- Substrate Reduction Therapy (SRT)
- Stem Cell Therapy
- Gene Therapy
- Supportive Care
By Disease Type
- Gaucher Disease
- Fabry Disease
- Pompe Disease
- Mucopolysaccharidosis (MPS)
- Niemann-Pick Disease
- Others
By Route of Administration
- Oral
- Intravenous
- Others
By End-User
- Hospitals
- Specialty Clinics
- Homecare
- Research Institutes
Future Outlook
The lysosomal storage disease treatment market is poised for considerable evolution over the next decade. Breakthroughs in gene editing and CRISPR-based therapies may offer potential cures rather than lifelong symptom management. Market expansion will be supported by growing awareness, enhanced global screening efforts, and the introduction of more affordable therapeutic options. As innovation continues to unfold, the focus will increasingly shift toward accessibility and personalized care, promising a brighter future for those affected by these rare conditions.
Buy this Premium Research Report@https://www.visionresearchreports.com/report/checkout/41753
You can place an order or ask any questions, please feel free to contact
sales@visionresearchreports.com| +1 650-460-3308
