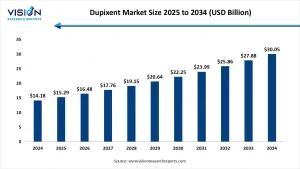
The global dupixent market size was accounted at around USD 14.18 billion in 2024 and it is projected to hit around USD 30.05 billion by 2034, growing at a CAGR of 7.80% from 2025 to 2034
Get a Sample@https://www.visionresearchreports.com/report/sample/41759
Dupixent Market Overview
Dupixent (dupilumab) is a monoclonal antibody developed by Sanofi and Regeneron, primarily used to treat chronic inflammatory conditions such as atopic dermatitis, asthma, and chronic rhinosinusitis with nasal polyps. As a biologic therapy that targets the IL-4 and IL-13 signaling pathways, Dupixent has become a transformative treatment in the immunology space. Its ability to address conditions with high unmet needs has contributed to widespread adoption and growing market demand.
With increasing global awareness of allergic and eosinophilic conditions and improved access to biologics, Dupixent is gaining traction not only in developed countries but also in emerging markets where regulatory pathways are evolving.
Dupixent Market Growth
The Dupixent market is experiencing robust growth, with its global valuation reaching approximately USD 14.18 billion in 2024. This figure is projected to more than double by 2034, hitting USD 30.05 billion, supported by consistent clinical efficacy and label expansions into additional indications. This growth trajectory reflects the confidence of healthcare providers and patients in the drug’s safety and long-term benefits.
A compound annual growth rate (CAGR) of 7.80% from 2025 to 2034 is anticipated due to multiple contributing factors, including an expanding pipeline of inflammatory diseases, aging populations, rising allergy prevalence, and increased patient access to advanced biologic therapies in outpatient and specialty care settings.
Dupixent Market Dynamics
Drivers
- Rising prevalence of chronic inflammatory and allergic diseases
- High clinical success rate and FDA approvals for multiple indications
- Increased awareness and diagnosis of atopic and eosinophilic conditions
Opportunities
- Geographic expansion into Asia-Pacific and Latin America
- Ongoing clinical trials for new indications
- Use in combination therapies for severe or refractory cases
Challenges
- High cost of biologics and reimbursement variability across regions
- Emergence of biosimilars and alternative therapies post-patent expiry
- Stringent regulatory pathways for newer indications
Applications in the Market
Dupixent is widely used in the treatment of various chronic inflammatory conditions where traditional therapies often fall short. It plays a crucial role in managing moderate-to-severe atopic dermatitis, especially in patients who do not respond well to topical treatments. In the field of respiratory health, Dupixent is prescribed for moderate-to-severe asthma, particularly in individuals with an eosinophilic phenotype, helping to reduce flare-ups and improve lung function.
Another important application of Dupixent is in treating chronic rhinosinusitis with nasal polyps. It helps shrink polyps and significantly improves nasal airflow and breathing, enhancing the quality of life for affected individuals. Additionally, Dupixent is approved to treat rare eosinophilic conditions like eosinophilic esophagitis in both adults and adolescents, offering a targeted and effective option where limited therapies previously existed.
Case Study Dupixent in Pediatric Atopic Dermatitis
A leading dermatology center in Germany introduced Dupixent therapy to pediatric patients aged 6–11 suffering from severe atopic dermatitis. Within 12 weeks, over 60% of patients achieved a 75% reduction in EASI scores, with significant improvement in sleep and quality of life. Minimal side effects and strong patient adherence further reinforced the drug’s safety profile in pediatric use. This success prompted wider adoption in European clinical guidelines.
Read More:https://www.heathcareinsights.com/herbal-supplements-market/
Dupixent Market Trends
- Expansion into New Indications: Dupixent is being tested in clinical trials for conditions like eosinophilic esophagitis, chronic spontaneous urticaria, and COPD, broadening its potential patient base.
- Pediatric Approvals Driving Volume Growth: Regulatory approvals for pediatric use in atopic dermatitis and asthma are driving a significant increase in prescription volumes worldwide.
- Improved Payer Access and Insurance Coverage: Increased awareness and insurance support for biologics are helping more patients afford and adhere to Dupixent therapy.
- Shift Toward Subcutaneous Self-Administration: Convenient self-injection formats are enhancing patient compliance and reducing the burden on healthcare systems.
Top Companies in Dupixent Market
- Sanofi S.A.
- Regeneron Pharmaceuticals, Inc.
- GlaxoSmithKline plc (GSK)
- Novartis AG
- AstraZeneca plc
- Eli Lilly and Company
- AbbVie Inc.
- Amgen Inc.
- F. Hoffmann-La Roche Ltd.
Market Segmentation
By Indication
- Atopic Dermatitis (AD)
- Asthma
- Chronic Rhinosinusitis with Nasal Polyps (CRSwNP)
- Chronic Obstructive Pulmonary Disease (COPD)
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Others
By Regional
- North America
- U.S.
- Canada
- Mexico
- Europe
- UK
- Germany
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Asia Pacific
- Japan
- China
- India
- Australia
- South Korea
- Thailand
- Latin America
- Brazil
- Argentina
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
Future Outlook
The future of the Dupixent market looks highly promising, with continued momentum from regulatory approvals, research expansion, and growing clinician confidence. The anticipated entry into new disease segments and broader demographic use will further solidify Dupixent’s status as a first-line biologic in immunology. Strategic partnerships, pricing reforms, and biosimilar management will play pivotal roles in shaping the next phase of market evolution.
Buy this Premium Research Report@https://www.visionresearchreports.com/report/checkout/41759
You can place an order or ask any questions, please feel free to contact
sales@visionresearchreports.com| +1 650-460-3308
