The global veterinary pharmacovigilance market size was accounted at USD 986.93 million in 2024 and it is projected to hit around USD 3,204.88 million by 2034, growing at a CAGR of 12.5% from 2025 to 2034.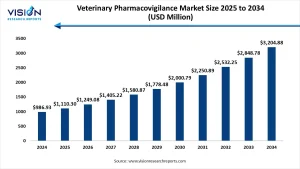
Veterinary Pharmacovigilance Market Overview
The veterinary pharmacovigilance market plays a crucial role in the continuous assessment of drug safety in animals. As veterinary care expands globally, there is a growing need to monitor adverse drug reactions, medication errors, and overall pharmacological effectiveness in animals. Veterinary pharmacovigilance systems help ensure the safety and efficacy of veterinary products used in livestock, companion animals, and exotic species. With the increased focus on animal health, food safety, and public health, regulatory agencies and pharmaceutical companies are investing in robust pharmacovigilance frameworks.
Get a Sample@https://www.visionresearchreports.com/report/sample/40183
Veterinary Pharmacovigilance Market Growth
The market is experiencing robust growth due to the increasing prevalence of zoonotic diseases and the rising demand for veterinary drugs. Governments and regulatory bodies across the globe are placing a stronger emphasis on drug safety surveillance, especially with the rising consumption of animal-derived food products. As a result, veterinary healthcare systems are evolving to implement real-time monitoring tools and AI-integrated pharmacovigilance platforms that help in early detection and reporting of adverse events.
Additionally, the growing awareness of responsible pet ownership and higher spending on companion animal care have fueled demand for safer veterinary drugs. Pharmaceutical companies are being mandated to provide post-market surveillance data, and this trend is pushing the need for structured and compliant pharmacovigilance processes.
Veterinary Pharmacovigilance Market Trends
- Digital Integration and AI-Based Monitoring: AI and machine learning tools are being integrated into veterinary pharmacovigilance for real-time detection of adverse drug reactions and predictive analytics.
- Global Harmonization of Regulations: Efforts are underway to align veterinary pharmacovigilance practices globally, improving reporting consistency and regulatory compliance across borders.
- Increased Emphasis on Livestock Pharmacovigilance: Due to food safety concerns, there’s growing focus on monitoring drug residues and adverse reactions in livestock, particularly in poultry, cattle, and swine.
- Veterinary Telehealth Expansion: With the rise of tele-veterinary consultations, remote reporting of adverse drug events is becoming more streamlined and widely adopted.
Veterinary Pharmacovigilance Market Dynamics
Drivers
- Rising incidences of zoonotic diseases
- Increasing global animal health expenditure
- Growing demand for safe animal-derived food products
Opportunities
- Integration of big data and real-time analytics
- Expansion into emerging markets with high livestock populations
- Development of mobile apps and online reporting systems
Challenges
- Lack of standardization in reporting procedures globally
- Limited awareness among veterinarians and farmers in developing regions
- Data privacy and regulatory compliance complexities
What is Veterinary Pharmacovigilance and Why It Matters
Veterinary pharmacovigilance is the science and activity of monitoring, detecting, and assessing the safety of veterinary medicines after they are released into the market. This includes keeping track of side effects, allergic reactions, and unexpected outcomes in animals treated with drugs or vaccines.
It matters because animals whether pets or livestock need safe treatments just like humans do. Unsafe or unmonitored drug use in animals can lead to illness, death, drug resistance, and even affect food safety when it comes to meat, milk, or eggs. By ensuring drugs are working as intended and without harm, pharmacovigilance protects animal welfare, public health, and the credibility of veterinary medicine.
Why This Market is Growing So Fast
The veterinary pharmacovigilance market is expanding quickly for several key reasons
- More People Are Owning Pets: As more households bring pets into their homes, the demand for veterinary care and safe drugs grows.
- Stricter Regulations: Governments are now requiring more detailed tracking of how drugs perform after they are sold, especially for food-producing animals.
- Animal Disease Concerns: The rise in zoonotic diseases (which spread between animals and humans) has made it important to closely monitor veterinary medicines.
- Advanced Technology: Digital tools, artificial intelligence, and mobile apps have made it easier to report and analyze adverse drug reactions in animals.
Different Types of Animal Drugs and Users
Types of Veterinary Drugs
- Vaccines – Prevent infections like rabies or parvovirus.
- Anti-infectives – Treat bacterial, viral, or fungal infections.
- Parasiticides – Kill or repel parasites like fleas, ticks, or worms.
- Pain Relievers and Anti-inflammatory Drugs – Help manage pain and reduce swelling.
- Hormones and Growth Regulators – Used in livestock to enhance productivity.
Main Users
- Veterinarians – Primary prescribers and reporters of drug safety issues.
- Livestock Farmers – Use drugs for herd health and productivity.
- Pet Owners – Administer medications for conditions like arthritis, skin allergies, or anxiety.
- Animal Health Companies – Develop drugs and monitor their post-market performance.
- Regulatory Authorities – Oversee drug approvals and manage adverse event databases.
Where Veterinary Drug Safety is Used in Real Life
Veterinary pharmacovigilance plays a role in many everyday situations, such as
- At a Pet Clinic: A dog treated for arthritis experiences a side effect like vomiting. The vet reports this to the drug safety database to track if others have the same reaction.
- On a Farm: A batch of antibiotics used on dairy cattle causes unexpected illness. The farmer notifies a vet, who investigates and reports it to authorities.
- In a Laboratory: A new vaccine is being monitored in post-approval studies to detect any long-term side effects in treated animals.
- In Food Safety Testing: Authorities ensure meat and milk products don’t contain harmful drug residues by monitoring how livestock drugs are used and metabolized.
Applications in the Market
Veterinary pharmacovigilance plays a vital role across various sectors of animal healthcare. In livestock farming, it ensures that drugs used on food-producing animals like cattle, poultry, and swine do not leave behind harmful residues, helping to maintain food safety standards. For companion animals, pharmacovigilance helps track and manage adverse reactions to commonly used treatments, ensuring the well-being of pets such as dogs and cats. Regulatory bodies like the FDA in the U.S. and the EMA in Europe rely on pharmacovigilance systems to enforce compliance, requiring drug manufacturers and veterinarians to report any safety concerns promptly. Additionally, veterinary clinical trials depend on post-market surveillance data to evaluate the long-term performance of drugs, aiding future research and development efforts. These applications collectively support safer, more effective veterinary care across species and regions.
Case Study EU Veterinary Pharmacovigilance System
The European Medicines Agency (EMA) implemented the Union Pharmacovigilance Database for veterinary medicines in 2022. This centralized system allows for easier reporting, analysis, and risk assessment of adverse events across EU member states. As a result, regulatory authorities have improved their responsiveness to safety concerns, and animal health companies benefit from more transparent feedback loops. The model is now being considered for replication in other regions.
Read More:https://www.heathcareinsights.com/single-use-pump-market/
Top Companies in Veterinary Pharmacovigilance Market
- ArisGlobal
- Accenture
- Ennov
- Sarjen Systems Pvt. Ltd.
- Pharsafer Associates Limited
- Knoell
- Biologit
- Indivirtus
- Azierta Contract Science Support Consulting
- Oy Medfiles Ltd.
Want custom data? Click here: https://www.visionresearchreports.com/report/customization/40183
Market Segmentations
By Solution
- Software
- Services
By Product
- Biologics
- Anti-infectives
- Other Product
By Type
- In-house
- Contract Outsourcing
By Animal Type
- Dogs
- Cats
- Other Animal Types
By Regional
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- Japan
- China
- India
- Australia
- South Korea
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- Rest of MEA
Future Outlook
The future of the veterinary pharmacovigilance market lies in the seamless integration of digital tools, automated reporting, and global regulatory harmonization. As more countries adopt strict pharmacovigilance frameworks, the industry is expected to grow steadily, offering lucrative opportunities to technology providers, CROs, and pharmaceutical companies. The increasing focus on “One Health” which ties together human, animal, and environmental health will further bolster investment in veterinary pharmacovigilance systems worldwide.
Buy this Premium Research Report@https://www.visionresearchreports.com/report/checkout/40183
You can place an order or ask any questions, please feel free to contact
sales@visionresearchreports.com| +1 650-460-3308
