The Asia Pacific clinical trials market size was accounted at around USD 16.85 billion in 2024 and it is projected to hit around USD 38.10 billion by 2034, growing at a CAGR of 8.5% from 2025 to 2034.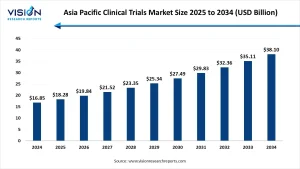
Get a Sample@https://www.visionresearchreports.com/report/sample/41755
Asia Pacific Clinical Trials Market Overview
The Asia Pacific clinical trials market was valued at approximately USD 16.85 billion in 2024 and is projected to reach USD 38.10 billion by 2034, expanding at a CAGR of 8.5% during the forecast period. Countries like China, India, South Korea, and Japan are emerging as hotspots due to their large patient pools, skilled professionals, and cost-effective operations. Increasing investments from global pharma and biotech firms are further fueling regional growth.
This region has become an attractive destination for clinical research due to favorable regulatory reforms, growing disease burden, and the expansion of R&D activities. Additionally, improvements in infrastructure and digital transformation in healthcare are supporting the effective management and monitoring of clinical trials.
Asia Pacific Clinical Trials Market Growth
The market is experiencing strong momentum as pharmaceutical and biotechnology companies increasingly outsource their clinical research activities to Asia Pacific to save on costs and shorten trial timelines. The availability of diverse and treatment-naïve populations also contributes to faster patient recruitment, a critical factor in the success of clinical trials.
Furthermore, supportive government initiatives and collaboration with international CROs (Contract Research Organizations) are expanding the scope of trials from simple therapeutic assessments to complex, multi-phase studies. With rapid urbanization and increased access to healthcare services, the region is well-positioned for sustainable growth in the clinical research domain.
What is the Asia Pacific Clinical Trials Market?
The Asia Pacific Clinical Trials Market is all about the testing of new medicines, vaccines, and medical devices in countries across Asia and the Pacific region—like India, China, Japan, South Korea, Australia, and others.
Before a new drug or treatment can be sold to the public, it must go through clinical trials to make sure it is safe and works well. These trials are done with real people under the guidance of doctors and researchers.
What’s Next for the Market?
The future of the Asia Pacific clinical trials market looks very promising. As more global pharmaceutical companies look to this region for faster and more affordable trials, the market is expected to grow even more in the coming years.
- More use of technology: Clinical trials will rely more on digital tools like mobile apps, artificial intelligence (AI), and remote monitoring to make the process faster and easier for both doctors and patients.
- Focus on personalized medicine: Instead of one-size-fits-all treatments, future trials will test medicines that are tailored to a person’s genes, age, or lifestyle.
- Stronger global partnerships: More international companies will team up with local hospitals and research centers in Asia to run large and complex trials.
- Better regulations and faster approvals: Governments in Asia are working to improve trial rules, so new treatments can be tested and approved more quickly and safely.
Asia Pacific Clinical Trials Market Dynamics
Drivers
- Increasing demand for cost-effective clinical research services
- Rising prevalence of chronic and infectious diseases
- Expansion of global pharmaceutical and biotech R&D investments
Opportunities
- Adoption of digital technologies in trial monitoring and data collection
- Emergence of personalized medicine and genomics-based trials
- Rising interest from foreign sponsors in Asia Pacific trial sites
Challenges
- Diverse regulatory frameworks across countries
- Ethical concerns and lack of awareness in rural regions
- Delays in patient recruitment due to cultural and language barriers
Applications in the Market
Clinical trials in the Asia Pacific region play a big role in improving healthcare by testing and developing new medical solutions. One of the main uses is to check if new drugs and vaccines are safe and effective before they can be approved for public use. This is especially important for serious diseases like cancer, diabetes, heart conditions, and neurological disorders, where better treatments are constantly needed.
These trials also help validate medical devices and diagnostic tools, making sure they work correctly and provide accurate results. In recent years, there has been a growing focus on personalized medicine, which means creating treatments that are tailored to specific groups of people based on their genetics, lifestyle, or region. Clinical trials help study how different populations respond to these new treatments.
Real-World Case Study China’s Rapid COVID-19 Vaccine Trials
During the COVID-19 pandemic, China conducted multiple rapid vaccine trials, including Sinovac and Sinopharm, with impressive turnaround times. The trials involved thousands of participants across multiple phases and were instrumental in the country’s early vaccine deployment. This showcased Asia Pacific’s ability to manage high-volume, multi-center trials under urgent timelines, reinforcing its role in global clinical research.
Key Market Trends
- Rise of Decentralized Trials: Virtual clinical trials and remote monitoring are becoming more common, reducing dependency on physical trial sites and improving patient engagement.
- Growing Adoption of AI & Big Data: AI-based platforms are being used to identify ideal candidates, predict outcomes, and optimize data management in trials, boosting efficiency.
- Regulatory Reforms Supporting Growth: Countries like China and India are simplifying trial approval processes and aligning with global regulatory standards to attract more trials.
- Increased Focus on Rare and Chronic Diseases: There’s a noticeable shift toward trials targeting rare disorders, oncology, and chronic conditions, reflecting global R&D priorities.
Read More:https://www.heathcareinsights.com/u-s-mhealth-apps-market/
Top Companies in Asia Pacific Clinical Trials Market
- IQVIA Inc.
- Parexel International Corporation
- ICON plc
- Syneos Health
- PPD (part of Thermo Fisher Scientific)
- Covance Inc. (a Labcorp company)
- Charles River Laboratories
- Pharmaceutical Product Development LLC
- WuXi AppTec
- Novotech
- SGS SA
- PRA Health Sciences
- Clinipace
- GVK Biosciences
- Asia CRO
Market Segmentation
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional Trials
- Observational Trials
- Expanded Access Trials
By Indication
- Autoimmune/Inflammation
- Rheumatoid Arthritis
- Multiple Sclerosis
- Osteoarthritis
- Irritable Bowel Syndrome (IBS)
- Others
- Pain Management
- Chronic Pain
- Acute Pain
- Oncology
- Blood Cancer
- Solid Tumors
- Others
- CNS Conditions
- Epilepsy
- Parkinson’s Disease (PD)
- Huntington’s Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle Regeneration
- Others
- Diabetes
- Obesity
- Cardiovascular Diseases
- Others
By Indication by Study Design
- Autoimmune/Inflammation
- Interventional Trials
- Observational Trials
- Expanded Access Trials
- Pain Management
- Interventional Trials
- Observational Trials
- Expanded Access Trials
- Oncology
- Interventional Trials
- Observational Trials
- Expanded Access Trials
- CNS Conditions
- Interventional Trials
- Observational Trials
- Expanded Access Trials
- Diabetes
- Interventional Trials
- Observational Trials
- Expanded Access Trials
- Obesity
- Interventional Trials
- Observational Trials
- Expanded Access Trials
- Cardiovascular Diseases
- Interventional Trials
- Observational Trials
- Expanded Access Trials
- Others
- Interventional Trials
- Observational Trials
- Expanded Access Trials
By Service
- Protocol Designing
- Site Identification
- Patient Recruitment
- Laboratory Services
- Analytical Testing Services
- Clinical Trial Data Management Services
- Others
By Sponsor
- Pharmaceutical & Biopharmaceutical Companies
- Medical Device Companies
- Others
By Regional
- Asia Pacific
- Japan
- China
- India
- Thailand
- South Korea
- Australia
Future Outlook
The Asia Pacific clinical trials market is set to witness continued growth due to robust government support, digital healthcare advancements, and increased foreign direct investment. Strategic partnerships between regional research institutes and global pharma giants will foster innovation and bring novel therapies to market faster. The focus will likely shift toward personalized treatments, AI-driven trial designs, and precision medicine as the region strengthens its position in the global clinical trials ecosystem.
Buy this Premium Research Report@https://www.visionresearchreports.com/report/checkout/41755
You can place an order or ask any questions, please feel free to contact
sales@visionresearchreports.com| +1 650-460-3308
